
@ShahidNShah

Platelet-rich plasma (PRP) therapy concentrates autologous platelets and growth factors to modulate synovial inflammation, support chondrocyte activity, and promote soft‑tissue repair in canine joints. Evidence shows reduced lameness scores, pain on manipulation, and improved range of motion in elbows and stifles. The procedure is minimally invasive, with a favorable safety profile versus repeated corticosteroid injections. Indications include osteoarthritis and ligament or tendon disease. Yet patient selection, protocol standardization, and expected outcomes merit closer scrutiny.
Platelet-rich plasma (PRP) is an autologous blood product concentrated to contain supraphysiologic levels of platelets and their bioactive components, used to modulate inflammation and promote tissue repair in dogs. Derived from a venous blood draw, PRP is prepared by differential centrifugation to enrich cellular components, chiefly platelets, while variably including leukocytes and minimal erythrocytes. Upon activation—via calcium chloride, thrombin, or collagen exposure—platelets degranulate, releasing growth factors such as PDGF, TGF-β, VEGF, IGF-1, and EGF, and synthesizing lipid mediators that influence synovium, cartilage, tendon, and ligament microenvironments.
These biological properties act locally: growth factors bind resident cell receptors, enhancing chemotaxis, angiogenesis, fibroblast proliferation, and extracellular matrix synthesis while attenuating proinflammatory cytokines (e.g., IL-1β, TNF-α) through NF-κB pathway modulation. Fibrin scaffolding provides provisional matrix for cell migration. Leukocyte content can be tailored; leukocyte-poor PRP reduces catabolic enzymes, whereas leukocyte-rich preparations may augment antimicrobial activity. Ultrasound-guided intra-articular or peri-tendinous administration optimizes delivery precision.
Platelet-rich plasma (PRP) is being applied intra-articularly to reduce osteoarthritis pain by modulating synovial inflammation, improving chondrocyte metabolism, and supporting extracellular matrix turnover. In peri-ligamentous and paratendinous injections, PRP supplies concentrated growth factors (e.g., PDGF, TGF-β, IGF-1) that promote tenocyte proliferation, collagen I remodeling, and enthesis healing in cranial cruciate ligament and common tendon injuries. Veterinary practices offering PRP therapy for pets in Reno have reported improved lameness scores and joint range of motion with low adverse-event rates, though outcomes vary with PRP preparation, dosing interval, and lesion chronicity.
Although degenerative joint disease advances gradually, osteoarthritis pain in dogs arises from a well-defined cascade: cartilage fibrillation and loss of proteoglycans expose subchondral bone, osteophytes develop at joint margins, and synovitis amplifies nociception via inflammatory mediators (e.g., IL‑1β, TNF‑α, PGE2). Within this context, platelet-rich plasma (PRP) is posited to attenuate pain by reducing inflammation and modulating joint biochemistry. Concentrated platelets release growth factors (TGF‑β, PDGF, IGF‑1) and anti-inflammatory cytokines that downregulate catabolic pathways in chondrocytes, limit MMP activity, and stabilize the synovial environment, supporting joint health.
Intra-articular PRP can decrease synovial effusion, improve limb use, and lower pain scores by diminishing peripheral sensitization at the joint capsule and subchondral interface. Ultrasound-guided delivery optimizes intra-articular distribution while minimizing soft-tissue irritation. Repeat treatments are individualized based on response.
Many canine soft-tissue injuries—partial cranial cruciate ligament (CCL) tears, collateral ligament sprains, supraspinatus tendinopathy, biceps tenosynovitis, and Achilles tendinopathy—derive from microtrauma, hypovascularity, and disorganized extracellular matrix. Platelet-rich plasma (PRP), concentrated in platelets and leukocyte-tailored, delivers PDGF, TGF-β, VEGF, and IGF-1 to stimulate tenocyte proliferation, type I collagen synthesis, and neovascularization within the paratenon and enthesis. Ultrasound-guided intratendinous or periligamentous injections target hypoechoic degenerative zones, reducing matrix metalloproteinase activity and normalizing collagen I:III ratios. In partial CCL and supraspinatus disease, PRP adjunctive to controlled rehabilitation enhances fiber alignment, reduces pain, and restores load-sharing across the stifle and shoulder, yielding improved joint mobility. While PRP does not induce cartilage regeneration directly, stabilizing periarticular tendons and ligaments decreases shear forces on articular surfaces, supporting longer-term joint health.
While enthusiasm can outpace data, the emerging evidence base for PRP in canine osteoarthritis shows measurable, though variable, clinical benefit. Across prospective cohorts and small randomized trials, veterinarians report reductions in lameness scores and pain on orthopedic exam, with improved dog joint function during gait analysis. Ultrasonography and radiography rarely show structural reversal, but owners and clinicians note functional gains lasting weeks to months. Adverse events are uncommon, supporting plasma therapy safety when asepsis and proper leukocyte profiles are maintained.
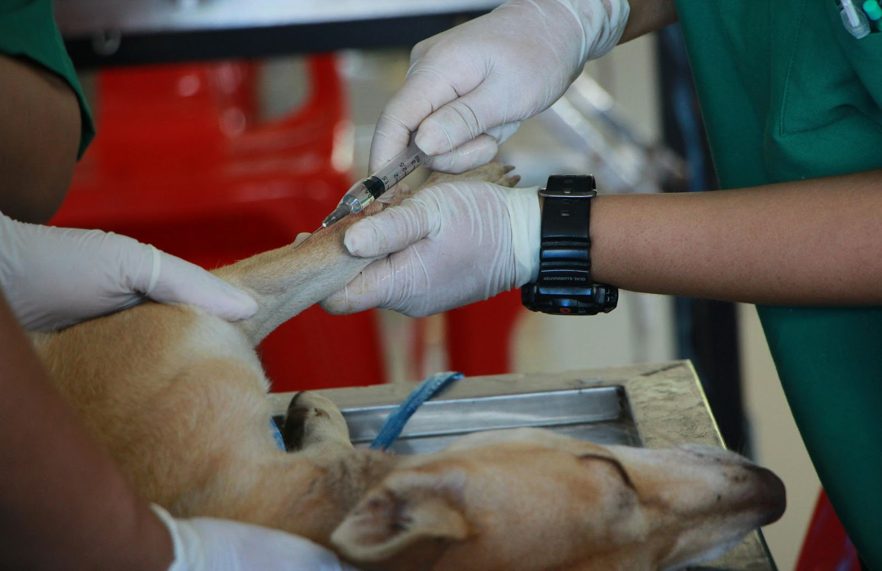
The process begins with a pre-procedure assessment, including orthopedic examination, gait analysis, and imaging to localize pathology and rule out contraindications. A peripheral venous blood draw is performed, followed by standardized centrifugation to concentrate platelets to 3–5× baseline while minimizing leukocyte contamination. Under sedation or local anesthesia, PRP is injected intra-articularly or peritendinously using aseptic technique with ultrasound guidance as indicated, and aftercare includes controlled activity, NSAID avoidance for 3–7 days, and follow-up evaluation of limb function.
How does a meticulous pre-procedure assessment reduce risk and optimize platelet-rich plasma (PRP) outcomes for dogs with osteoarthritis or joint pathology? It begins with a Full physical exam and baseline pain assessment to localize dysfunction and quantify disability. Orthopedic palpation evaluates joint effusion, crepitus, range of motion, and periarticular fibrosis; neurologic screening rules out radiculopathy or myelopathy that may mimic lameness. Imaging (radiography, and when indicated ultrasound or CT) confirms articular pathology, guides target selection, and identifies contraindications such as aggressive neoplasia or septic arthritis. Hematology and chemistry panels detect anemia, thrombocytopenia, coagulopathy, or systemic disease that could blunt response or increase risk.
With candidacy confirmed and target joints selected, attention shifts to atraumatic venipuncture and standardized processing to yield a therapeutic platelet dose. Venous access procedures typically use the cephalic or lateral saphenous vein; a 19–21G butterfly minimizes shear that activates platelets. Blood is collected into anticoagulant (ACD-A) at a 1:9 ratio, gently inverted to prevent microclotting. Sample preparation techniques prioritize sterility and time-to-spin under 30 minutes.
A two-step centrifugation is common: a soft spin (e.g., 200 g for 10 minutes) separates red cells; plasma and buffy coat are transferred without disturbing erythrocytes. A hard spin (e.g., 400–800 g for 5–10 minutes) pellets platelets. Excess platelet-poor plasma is decanted to achieve 3–5× baseline platelet concentration. Leukocyte content is tailored—leukocyte-poor for intra-articular use—to limit inflammatory cytokines.
Typically, intra-articular PRP administration proceeds under aseptic conditions with image guidance to ascertain accurate deposition. Sedation or light general anesthesia is selected based on joint, temperament, and anesthesia considerations. Skin is clipped, chlorhexidine-prepped, and draped. The injection location is confirmed by ultrasound or fluoroscopy: e.g., craniolateral approach to the stifle via the parapatellar recess, medial compartment of the elbow between the radial head and humeral condyle, or dorsomedial hip under distraction.
A careful appraisal of platelet-rich plasma (PRP) therapy in canine osteoarthritis emphasizes its autologous origin and generally favorable safety profile, while acknowledging defined risks and contraindications. Potential side effects are typically transient: post-injection flare (48–72 hours of increased lameness), synovitis with effusion, localized warmth, and mild discomfort at periarticular soft tissues. Hematoma or bruising can occur at venipuncture or injection sites; infection is rare with asepsis. Immune reactions are uncommon due to autologous use, yet sensitivity to anticoagulants or local anesthetics should be considered.
Contraindication considerations include septic arthritis, cellulitis over the intended portal, systemic infection, coagulopathy or thrombocytopenia, severe anemia, neoplasia within or near the target joint, uncontrolled endocrinopathies, and autoimmune polyarthritis until stabilized. Caution is advised in dogs receiving immunosuppressants or recent intra-articular steroids due to altered platelet function. Advanced structural collapse (end-stage osteoarthritis with subchondral bone exposure or significant osteophyte impingement) may limit clinical response. Pre-procedural CBC, coagulation profile, and imaging guide case selection and timing.
Compared with corticosteroid injections, nonsteroidal anti-inflammatory drugs (NSAIDs), and surgical interventions, platelet-rich plasma (PRP) targets joint pathology via biologic modulation rather than symptomatic suppression or structural alteration. In canine osteoarthritis, PRP delivers autologous growth factors that influence synoviocytes, chondrocytes, and subchondral bone remodeling, aiming to reduce synovitis and stimulate matrix repair. When Comparing PRP to traditional medications, evidence indicates longer dosing intervals and fewer systemic adverse effects relative to NSAIDs, and avoidance of cartilage catabolism associated with repeated intra-articular steroids.
Building on the biologic advantages over NSAIDs, steroids, and surgery, patient selection centers on dogs with clinical osteoarthritis confirmed by orthopedic exam and imaging (radiography ± ultrasound/CT) showing articular cartilage thinning, osteophytes, and synovitis, where pain and lameness persist despite optimized multimodal management (weight control, controlled exercise/rehab, omega-3 supplementation, and appropriately trialed NSAIDs or gabapentin/amantadine). Ideal candidates include skeletally mature dogs with stable comorbidities, intact cruciate function or well-compensated stifles post-stabilization, and focal pain localized to the hip, elbow, stifle, or carpus. Ideal age thresholds commonly fall in middle-aged to senior cohorts; however, younger adults with post-traumatic chondropathy may benefit.
Exclusions include end-stage bone-on-bone contact, severe varus/valgus deformity, uncontrolled immune-mediated polyarthritis, septic arthritis, neoplasia, or coagulopathy. Candidate fitness levels should allow light sedation and post-injection activity restriction; morbid obesity and poor conditioning reduce response. Bilateral disease and multifocal joints are acceptable when each joint has palpable effusion, reduced range of motion, and pain on compression or manipulation.
While fees vary by geography and clinic infrastructure, most platelet-rich plasma (PRP) programs for canine osteoarthritis are billed per joint and per session, reflecting costs for phlebotomy, kit/centrifuge processing, sedation, ultrasound-guided intra-articular injection, and recheck assessments. Typical treatment cost ranges cover unilateral elbows, stifles, or hips, with incremental pricing for bilateral sites. Packages may bundle initial consult, CBC to confirm platelet adequacy, and post-injection gait evaluations.
Although PRP can attenuate synovitis and modulate nociceptive signaling, outcomes are optimized when integrated with targeted rehabilitation, weight management, and environmental modification. A structured exercise regimen emphasizes low-impact, closed-chain movements to strengthen the quadriceps, hamstrings, and gluteals, stabilize the stifle and hip, and support thoracolumbar control. Hydrotherapy and controlled leash walks improve joint range of motion while limiting shear forces on articular cartilage. Progressive loading should track lameness scores and thigh circumference.
Caloric restriction to achieve a lean body condition reduces compressive forces across the coxofemoral, stifle, and elbow joints. High-protein, omega-3–enriched diets may mitigate inflammatory mediators. Evidence-based dietary supplement options include EPA/DHA, green-lipped mussel, undenatured type II collagen, and joint-protective glycosaminoglycans.
Environmental adjustments—non-slip flooring, ramps, elevated feeding, and supportive bedding—minimize repetitive microtrauma. Neuromuscular re-education, proprioceptive work (cavaletti rails, wobble boards), and manual therapy can normalize gait mechanics. Periodic rechecks align PRP timing with functional milestones and prevent overexertion.
Yes, depending on policy eligibility. Some insurers provide cost coverage for PRP therapy when deemed medically necessary by a veterinarian, with preauthorization. Coverage varies by plan, exclusions, deductibles, and limits; documentation of diagnosis, joint affected, and treatment protocol is required.
They should prioritize board-certified veterinarians experienced in PRP, verifying caseload, ultrasound-guided intra-articular technique, aseptic protocol, and biologics handling. Finding local veterinary specialists, evaluating veterinarian qualifications, requesting outcome data, sedation/analgesia plans, and post-procedure rehabilitation guarantees anatomically precise, evidence-based care with minimized iatrogenic risk.
Sedation is rarely required; anesthesia is uncommon. Most dogs tolerate venipuncture with minimal pain using proper preparation: atraumatic needles, limb stabilization, cephalic or jugular vein access, topical lidocaine, gentle restraint, and stress-reduction. Anxiolytics may be considered for fractious or highly anxious patients.
Yes, combination therapy is generally safe when protocols are sterile and doses standardized. Evidence suggests synergistic anti-inflammatory effects, cartilage matrix support, and improved mobility outcomes. As a minimally invasive procedure, intra-articular injections target synovium, subchondral bone interfaces, and periarticular soft tissues.
Improvement is indicated by increased mobility, smoother sit-to-stand shifts, normalized gait, reduced inflammation (less joint heat/swelling), and decreased analgesic need. Failure appears as persistent lameness, guarded range of motion, joint effusion, nocturnal restlessness, and unchanged or escalating pain behaviors despite activity modification.

Emergency eye care is critical in preserving vision in various situations. Eye injuries from accidents, chemical exposures, and sudden vision changes demand immediate attention. Foreign objects, …
Posted Sep 25, 2025 Ophthalmology
Connecting innovation decision makers to authoritative information, institutions, people and insights.
Medigy accurately delivers healthcare and technology information, news and insight from around the world.
Medigy surfaces the world's best crowdsourced health tech offerings with social interactions and peer reviews.
© 2026 Netspective Foundation, Inc. All Rights Reserved.
Built on Mar 6, 2026 at 2:19pm