
@ShahidNShah

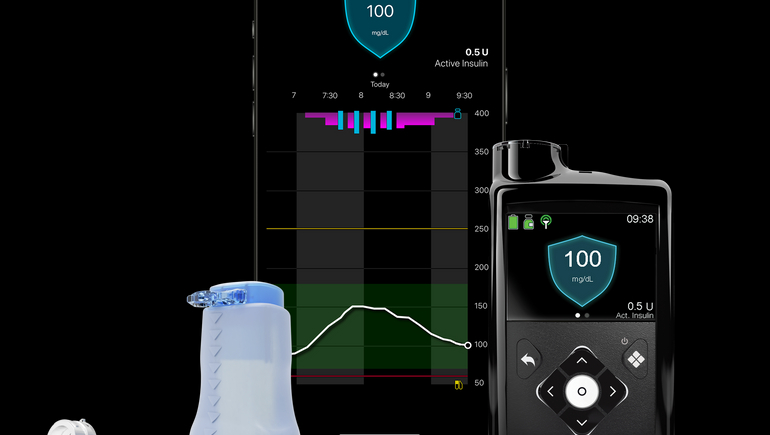
Due to regulatory issues at its diabetic section, Medtronic's MiniMed 780G insulin pump took longer than anticipated to receive U.S. clearance, taking place in April. The company is now turning on the gadget to help it recover from a four-year decline in diabetes business revenues and take market share away from competitors like Insulet and Tandem Diabetes Care. The American Diabetes Association (ADA) Scientific Sessions have been used by Medtronic to demonstrate how their recently approved MiniMed 780G pump can enhance the management of diabetes in kids. The results of a short clinical research were presented at the event by Medtronic, which revealed that adolescents using the pump can improve blood glucose management without carefully measuring carbohydrates.
Continue reading at medtechdive.com
With regard to pharmaceuticals and digital health, we are at an intriguing turning point. Many pharma companies are taking a step back to strategically analyse how they have performed in practice and …
Posted Jul 8, 2023 Pharma Industry Digital Health Pharmaceutical Services
Connecting innovation decision makers to authoritative information, institutions, people and insights.
Medigy accurately delivers healthcare and technology information, news and insight from around the world.
Medigy surfaces the world's best crowdsourced health tech offerings with social interactions and peer reviews.
© 2025 Netspective Foundation, Inc. All Rights Reserved.
Built on Jun 30, 2025 at 6:08am