
@ShahidNShah

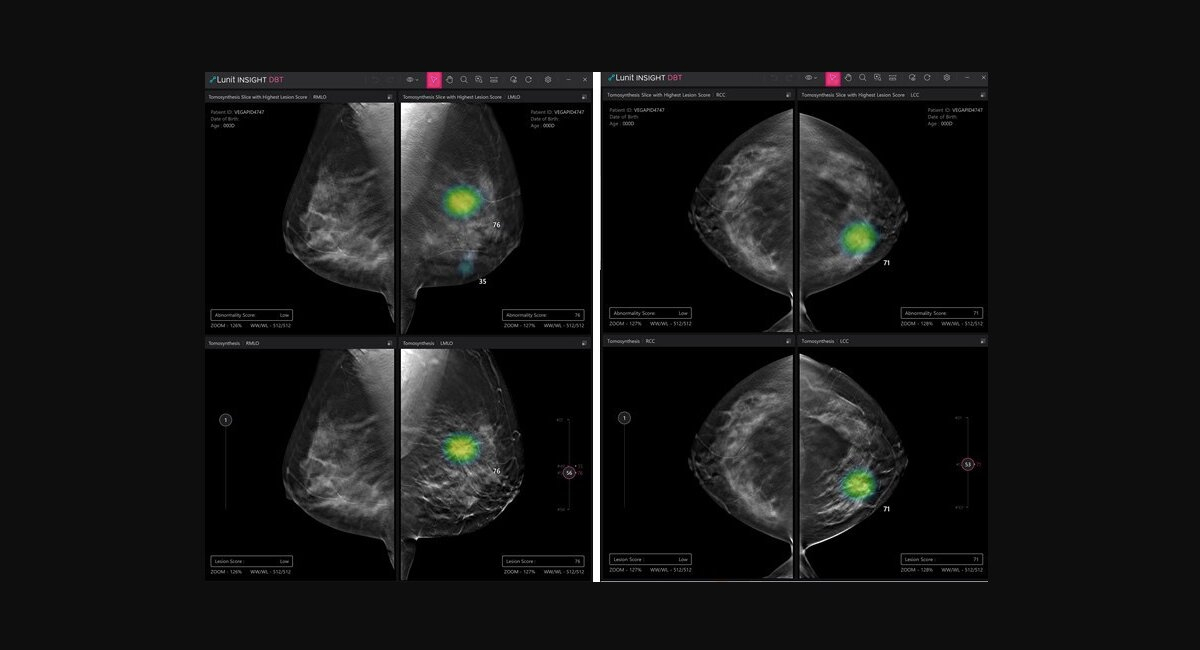
Also, Jolly Good has collaborated with Brigham and Women's Hospital to create medical education VR content. For its AI software for digital breast tomosynthesis (DBT) analysis, Lunit, a South Korean medical AI business, has been granted a CE certification under the most recent Medical Device Regulation in Europe. The software programme, known as Lunit INSIGHT DBT, analyses 3D images from DBT and enables rapid and precise breast cancer identification. Lunit announced its intention to launch the software product in Europe by the end of March in a press release, citing an increase in interest. Also, it stated that it would start the procedure for Lunit INSIGHT DBT FDA approval in the third quarter. In South Korea, the technology has already received early this year approval for commercialization. Jolly Good partners with Brigham and Women's Hospital to produce VR content for emergency treatment.
Continue reading at mobihealthnews.com
There's been a softening of healthcare organizations' confidence in the vendor, researchers say, with customers expressing concerns about a post-acquisition roadmap and dissatisfaction with …
Posted Mar 25, 2023 Revenue Cycle Management EMR / EHR
Connecting innovation decision makers to authoritative information, institutions, people and insights.
Medigy accurately delivers healthcare and technology information, news and insight from around the world.
Medigy surfaces the world's best crowdsourced health tech offerings with social interactions and peer reviews.
© 2025 Netspective Foundation, Inc. All Rights Reserved.
Built on Jul 18, 2025 at 12:24pm