
@ShahidNShah

The system was cleared for use among individuals 12 years old and up with Type 1 or Type 2 diabetes on multiple-dose injection therapy.
The system was cleared for use among individuals 12 years old and up with Type 1 or Type 2 diabetes who use multiple-dose injection therapy.
Included in the Bigfoot Unity System are two smartpen caps for both rapid- and long-acting insulin, a connected mobile app, an integrated FreeStyle Libre 2 Sensor from Abbott and a blood glucose meter.
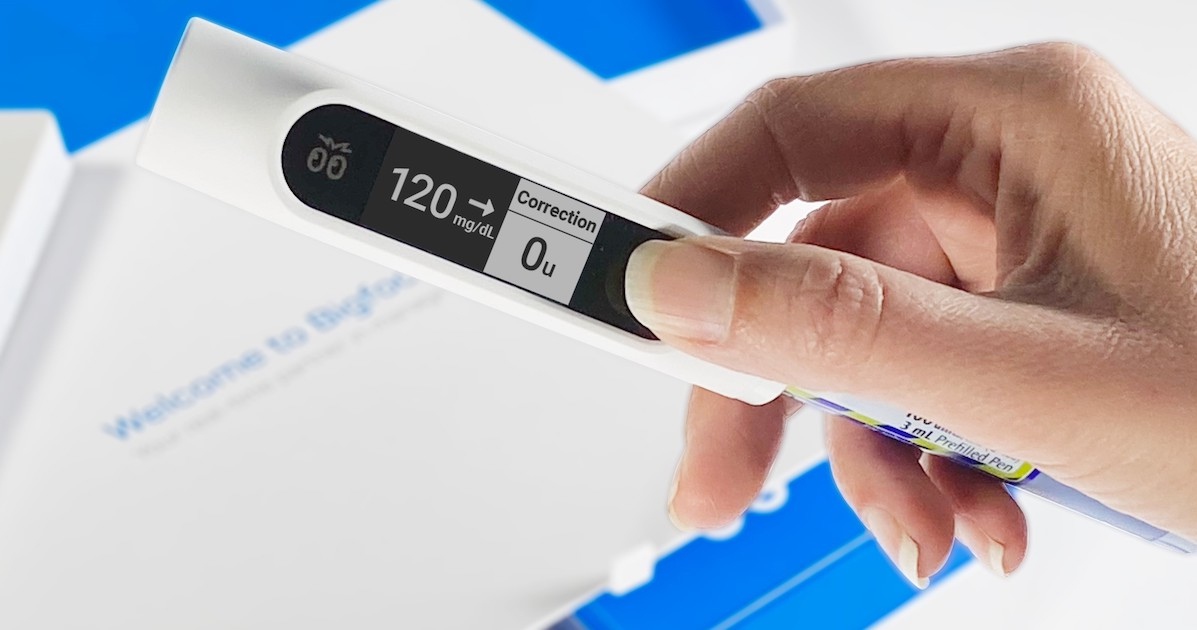
What sets Bigfoot’s system apart is the smartpen caps that take data from the FreeStyle Libre 2 continuous glucose monitor to give users insulin dose recommendations based on a physician’s instructions. To use the smartpen cap for rapid-acting insulin, users scan the FreeStyle Libre 2 sensor and the pen cap displays their current glucose value as well as any recommended correction doses.
The Unity System also provides reminders for long-acting insulin doses and notifications for when a person’s glucose level is too low. All of the data is stored and shared with the Bigfoot app for users and their healthcare providers to view their health history.
Additionally, Bigfoot’s smartpen caps are compatible with all major U.S. brands of rap
Continue reading at mobihealthnews.com
In a new, forward-looking report from health technology company Philips, researchers predict that artificial intelligence (AI) will overtake telehealth as the top digital health investment in the next …
Posted May 12, 2021 Digital Health Innovation Telehealth Artificial Intelligence
Connecting innovation decision makers to authoritative information, institutions, people and insights.
Medigy accurately delivers healthcare and technology information, news and insight from around the world.
Medigy surfaces the world's best crowdsourced health tech offerings with social interactions and peer reviews.
© 2024 Netspective Media LLC. All Rights Reserved.
Built on Apr 26, 2024 at 6:14am